Article Synopsis
- - The study presents a method for identifying single B cells that produce specific antibodies by using antigen-conjugated fluorescent beads, specifically focusing on the Folate Receptor alpha (FRα) as a model antigen.
- - Researchers used a mouse B cell line with a chimeric antibody targeting FRα to sort and analyze single antibody-expressing cells, allowing them to isolate and clone the antibody's genetic components.
- - The method proved effective in identifying antigen-specific B cells from mixed human immune cell populations, successfully generating monoclonal antibodies that recognize specific cancer-related antigens, paving the way for deeper insights into individual immune responses.
Category Ranking
98%
Total Visits
921
Avg Visit Duration
2 minutes
Citations
20
Article Abstract
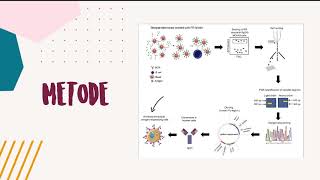
Selection of single antigen-specific B cells to identify their expressed antibodies is of considerable interest for evaluating human immune responses. Here, we present a method to identify single antibody-expressing cells using antigen-conjugated fluorescent beads. To establish this, we selected Folate Receptor alpha (FRα) as a model antigen and a mouse B cell line, expressing both the soluble and the membrane-bound forms of a human/mouse chimeric antibody (MOv18 IgG1) specific for FRα, as test antibody-expressing cells. Beads were conjugated to FRα using streptavidin/avidin-biotin bridges and used to select single cells expressing the membrane-bound form of anti-FRα. Bead-bound cells were single cell-sorted and processed for single cell RNA retrotranscription and PCR to isolate antibody heavy and light chain variable regions. Variable regions were then cloned and expressed as human IgG1/k antibodies. Like the original clone, engineered antibodies from single cells recognized native FRα. To evaluate whether antigen-coated beads could identify specific antibody-expressing cells in mixed immune cell populations, human peripheral blood mononuclear cells (PBMCs) were spiked with test antibody-expressing cells. Antigen-specific cells could comprise up to 75% of cells selected with antigen-conjugated beads when the frequency of the antigen-positive cells was 1:100 or higher. In PBMC pools, beads conjugated to recombinant antigens FRα and HER2 bound antigen-specific anti-FRα MOv18 and anti-HER2 Trastuzumab antibody-expressing cells, respectively. From melanoma patient-derived B cells selected with melanoma cell line-derived protein-coated fluorescent beads, we generated a monoclonal antibody that recognized melanoma antigen-coated beads. This approach may be further developed to facilitate analysis of B cells and their antibody profiles at the single cell level and to help unravel humoral immune repertoires.
Download full-text PDF |
Source |
|---|---|
| http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5876289 | PMC |
| http://dx.doi.org/10.3389/fimmu.2018.00493 | DOI Listing |
Publication Analysis
Top Keywords
Similar Publications
A Targeted Integration-Based CHO Cell Platform for Simultaneous Antibody Display and Secretion.
Antibodies (Basel)
April 2025
Bioprocessing Technology Institute (BTI), Agency for Science, Technology and Research (A*STAR), 20 Biopolis Way, #03-01 Centros, Singapore 138668, Singapore.
Objective: We developed a targeted integration-based CHO cell platform for simultaneous antibody display and secretion, enabling a streamlined transition from antibody library screening to production without requiring the re-cloning of antibody genes.
Methods: The platform consists of a CHO master cell line with a single-copy landing pad, a helper vector expressing FLPe recombinase, and bi-functional targeting vectors. Recombinase-mediated cassette exchange was utilized to integrate targeting vectors into the landing pad.
Identification of cellular signatures associated with chinese hamster ovary cell adaptation for secretion of antibodies.
Comput Struct Biotechnol J
December 2024
Cell Culture and Fermentation Sciences, BioPharmaceutical Development, AstraZeneca, Cambridge UK.
The secretory capacity of Chinese hamster ovary (CHO) cells remains a fundamental bottleneck in the manufacturing of protein-based therapeutics. Unconventional biological drugs with complex structures and processing requirements are particularly problematic. Although engineered vector DNA elements can achieve rapid and high-level therapeutic protein production, a high metabolic and protein folding burden is imposed on the host cell.
View Article and Find Full Text PDFA novel dual-epigenetic inhibitor enhances recombinant monoclonal antibody expression in CHO cells.
Appl Microbiol Biotechnol
September 2024
School of Pharmacy, Xinxiang Medical University, Xinxiang, 453003, Henan, China.
Article Synopsis
- Epigenetic regulation is crucial for controlling cellular processes like growth and cell death, and small molecule modulators can fine-tune gene expression in these processes.
- The study found that the dual-HDAC/LSD1 inhibitor I-4 significantly boosted monoclonal antibody production in CHO cells, leading to nearly double the antibody titer despite causing cell cycle arrest.
- Results indicate that I-4 enhances protein expression by increasing histone acetylation and downregulating the HDAC5 gene, demonstrating a new approach to improve recombinant protein yields.
Chimeric HLA antibody receptor T cells for targeted therapy of antibody-mediated rejection in transplantation.
HLA
October 2023
Laboratori Experimental de Nefrologia i Trasplantament (LENIT), Fundació de Recerca Clinic Barcelona-Institut d'Investigacions Biomèdiques August Pi i Sunyer (FRCB-IDIBAPS), Barcelona, Spain.
The presence of donor-specific antibodies (DSA), mainly against HLA, increases the risk of allograft rejection. Moreover, antibody-mediated rejection (ABMR) remains an important barrier to optimal long-term outcomes after solid organ transplantation. The development of chimeric autoantibody receptor T lymphocytes has been postulated for targeted therapy of autoimmune diseases.
View Article and Find Full Text PDFThe development of single-chain antibody anchored on the BmE cell membrane to inhibit BmNPV infection.
J Invertebr Pathol
June 2023
State Key Laboratory of Silkworm Genome Biology, Southwest University, Chongqing 400715, China; Chongqing Key Laboratory of Microsporidia Infection and Prevention, Southwest University, Chongqing 400715, China; College of Life Sciences, Chongqing Normal University, Chongqing 401331, China. Electroni
Bombyx mori nucleopolyhedrovirus (BmNPV) poses a significant threat to sericulture production, and traditional sanitation practices remain the main strategy for controlling BmNPV infection. Although RNAi targeting BmNPV genes engineered into transgenic silkworms has shown to be a promising approach in reducing viral infection, it cannot block viral entry into host cells. Therefore, there is an urgent need to develop new effective prevention and control measures.
View Article and Find Full Text PDF